Non-precious bimetallic catalysts for selective dehydrogenation of an organic chemical hydride system†
Abstract
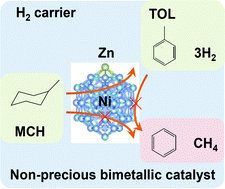
Methylcyclohexane (MCH)–toluene (TOL) chemical hydride cycles as hydrogen carrier systems are successful with the selective dehydrogenation of MCH to TOL, which has been achieved only using precious Pt-based catalysts. Herein, we report improved selectivity using non-precious metal nickel-based bimetallic catalysts, where the second metal occupies the unselective step sites.

 Please wait while we load your content...
Please wait while we load your content...