Transition-metal-free, ambient-pressure carbonylative cross-coupling reactions of aryl halides with potassium aryltrifluoroborates†
Abstract
We disclose an unprecedented transition-metal-free carbonylative cross coupling of aryl halides with potassium aryl trifluoroborates even at atmospheric pressure of carbon monoxide. This protocol is efficient, operationally simple, and shows wide scope with regard to both aryl halides and potassium aryl trifluoroborates containing a series of active functional groups.
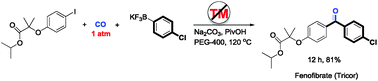

 Please wait while we load your content...
Please wait while we load your content...