A fused meso-aminoporphyrin: a switchable near-IR chromophore†
Abstract
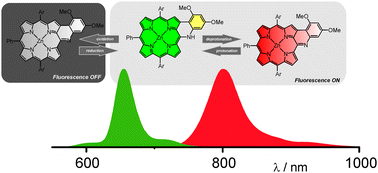
An aryl amine attached to the meso position of a porphyrin controls the π-delocalization using a redox process or a protonation/deprotonation centered at the meso-nitrogen. An easily accessible modulated motif affords a switchable near-IR chromophore as reflected by significant changes in absorption and fluorescence spectra.

 Please wait while we load your content...
Please wait while we load your content...