Vinylogy in nitronates: utilization of α-aryl conjugated nitroolefins as a nucleophile for a highly stereoselective aza-Henry reaction†
Abstract
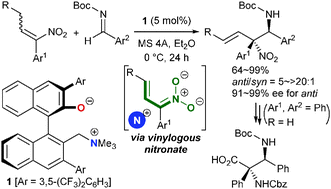
The vinylogous reactivity of α,β-disubstituted nitroolefins was uncovered through the facile generation of the corresponding α-substituted vinylogous nitronates and their use in the development of a highly diastereo- and enantioselective aza-Henry reaction with N-Boc aldimines under the catalysis of chiral ammonium betaines. The novel vinylogous nitronates undergo stereoselective bond formation at the sterically encumbered α-position exclusively, allowing the construction of contiguous tertiary–quaternary stereogenic carbon centers.

 Please wait while we load your content...
Please wait while we load your content...