σ-Alkenyl endo-palladacycle formation via regiospecific functionalisation of an unreactive NHC-tethered C(sp2)–H bond†
Abstract
An unusual cyclometallation reaction at palladium is described, which proceeds via C–H functionalisation of a vinylic C(sp2)–H bond tethered to an NHC ligand. The energetic balance between palladacycle formation and bis-NHC complexation has been found to be very subtle.
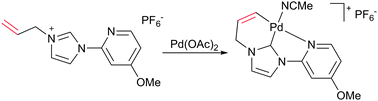
- This article is part of the themed collection: 2015 Emerging Investigators

 Please wait while we load your content...
Please wait while we load your content...