Olefins from biomass feedstocks: catalytic ester decarbonylation and tandem Heck-type coupling†
Abstract
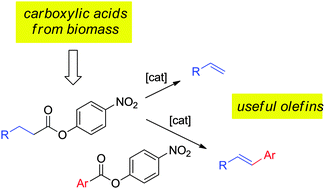
With the goal of avoiding the need for anhydride additives, the catalytic decarbonylation of p-nitrophenylesters of aliphatic carboxylic acids to their corresponding olefins, including commodity monomers like styrene and acrylates, has been developed. The reaction is catalyzed by palladium complexes in the absence of added ligands and is promoted by alkali/alkaline-earth metal halides. Combination of catalytic decarbonylation and Heck-type coupling with aryl esters in a single pot process demonstrates the viability of employing a carboxylic acid as a “masked olefin” in synthetic processes.

 Please wait while we load your content...
Please wait while we load your content...