(E)-α,β-unsaturated amides from tertiary amines, olefins and CO via Pd/Cu-catalyzed aerobic oxidative N-dealkylation†
Abstract
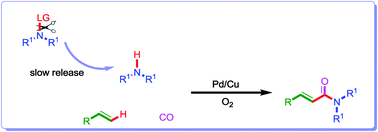
A novel Pd/Cu-catalyzed chemoselective aerobic oxidative N-dealkylation/carbonylation reaction has been developed. Tertiary amines are utilized as a “reservoir” of “active” secondary amines in this transformation, which inhibits the formation of undesired by-products and the deactivation of the catalysts. This protocol allows for an efficient and straightforward construction of synthetically useful and bioactive (E)-α,β-unsaturated amide derivatives from easily available tertiary amines, olefins and CO.

 Please wait while we load your content...
Please wait while we load your content...