Ni-Catalyzed α-arylation of esters and amides with phenol derivatives†
Abstract
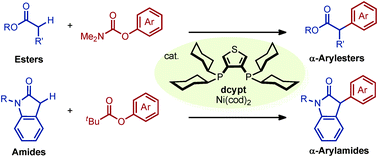
A nickel-catalyzed α-arylation of esters and amides with phenol derivatives has been accomplished. In the presence of our unique nickel catalyst, prepared in situ from Ni(cod)2, 3,4-bis(dicyclohexylphosphino)thiophene (dcypt), and K3PO4, various esters and amides undergo α-arylation with O-arylpivalates or O-arylcarbamates to afford the corresponding coupling products. The thus obtained α-aryl esters and amides are useful precursors of privileged motifs such as α-arylcarboxylic acids and β-arylamines.

 Please wait while we load your content...
Please wait while we load your content...