Thiol–ene immobilisation of carbohydrates onto glass slides as a simple alternative to gold–thiol monolayers, amines or lipid binding†
Abstract
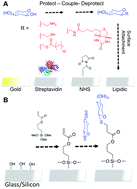
Carbohydrate arrays are a vital tool in studying infection, probing the mechanisms of bacterial, viral and toxin adhesion and the development of new treatments, by mimicking the structure of the glycocalyx. Current methods rely on the formation of monolayers of carbohydrates that have been chemically modified with a linker to enable interaction with a functionalised surface. This includes amines, biotin, lipids or thiols. Thiol-addition to gold to form self-assembled monolayers is perhaps the simplest method for immobilisation as thiolated glycans are readily accessible from reducing carbohydrates in a single step, but are limited to gold surfaces. Here we have developed a quick and versatile methodology which enables the use of thiolated carbohydrates to be immobilised as monolayers directly onto acrylate-functional glass slides via a ‘thiol–ene’/Michael-type reaction. By combining the ease of thiol chemistry with glass slides, which are compatible with microarray scanners this offers a cost effective, but also useful method to assemble arrays.


 Please wait while we load your content...
Please wait while we load your content...