Development and validation of ultra-high-performance liquid chromatography for the determination of sennoside A and sennoside B in laxatives based on optimal chromatographic parameters
Abstract
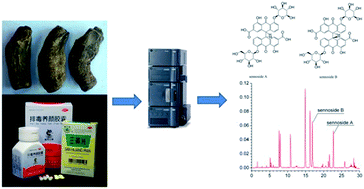
Sennoside A and sennoside B are the major bioactive components in laxative herbs like rhubarb, senna, etc. The previously reported techniques for the quantitative analysis of sennoside A and sennoside B have the disadvantages of defective peak purity and stability. This study investigates the influence of analytical parameters on the efficiency of separation of sennoside A and sennoside B in rhubarb by ultra-high-performance liquid chromatography (UHPLC). The chromatographic parameters of column temperature and flow rate have a non-linear relationship with the theoretical plate number and symmetry factor; the optimal column temperature was 30 °C and the optimal flow rate was 0.20 mL min−1. A new UHPLC analytical method was developed based on these optimal parameters. The chromatographic peak purity of sennoside A and sennoside B in rhubarb was satisfactory. This UHPLC-based analytical method was successfully applied for the quantitative determination of sennoside A and sennoside B in two sources of rhubarbs, Cassia angustifolia Vahl. and Paidu Yangyan capsule. There are remarkable content differences between sennoside A (more than 19 times) and sennoside B (more than 18 times) in the two species of rhubarb tested. In order to make the analytical method widely applicable, the analytical parameters of UHPLC have been converted into those of HPLC. In conclusion, the devised technique is suitable for the quality control of laxative herbs and diet drugs containing sennoside A and sennoside B.

 Please wait while we load your content...
Please wait while we load your content...