Analytical validation of a Biochip prototype for integrated analysis of AFP-IgM and SCCA-IgM serum biomarkers in patients with liver cirrhosis and hepatocellular carcinoma†
Abstract
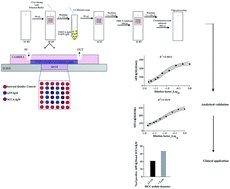
Aim: this study evaluates the analytical and clinical performances of a new technology, CompleXima HCC Biochip, for the simultaneous serum measurement of alpha-fetoprotein-IgM (AFP-IgM) and squamous cell carcinoma antigen-IgM (SCCA-IgM). Methods: AFP- and SCCA-IgM were measured by both ELISA and CompleXima HCC Biochip in 39 blood donors and in 174 patients (102 liver cirrhosis – LC – and 72 hepatocellular carcinoma – HCC). Results: the intra-assay coefficients of variation were lower than 12% and the inter-assay variations comprised between 14% and 21%. The linearity interval for the CompleXima HCC Biochip was 50–300 AU mL−1 for AFP-IgM and SCCA-IgM. The comparison between the prototype and the ELISA test was studied by using the Bland–Altman method and Passing–Bablok regression analyses. Passing–Bablok showed that the Biochip under-estimated AFP-IgM (Intercept A: −165.06; 95% CI: −313.11 to −51.32) and overestimated SCCA-IgM (Intercept A: 26.83; 95% CI: 14.47–35.86) with respect to ELISAs. Both biomarkers were higher in LC and HCC with respect to controls (p < 0.001) with no difference between LC and HCC (p = 0.864 for AFP-IgM and p = 0.214 for SCCA-IgM). The thresholds for AFP-IgM and SCCA-IgM were calculated by means of ROC curves, fixing the specificity at 95%. The sensitivity of AFP-IgM and SCCA-IgM associated with CompleXima in detecting patients with liver diseases was 47% and 46%, respectively. The combined evaluation of macrocomplexes with CompleXima in diagnosing HCC with respect to LC was associated with a sensitivity of 51.4% and a specificity of 48%. Conclusions: AFP-IgM and SCCA-IgM increase in chronic liver disease. The prototype CompleXima HCC Biochip allows their measurement with a good analytical reproducibility.

 Please wait while we load your content...
Please wait while we load your content...