An Fe3O4@SiO2@polypyrrole magnetic nanocomposite for the extraction and preconcentration of Cd(ii) and Ni(ii)†
Abstract
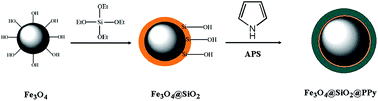
This work describes a novel Fe3O4@SiO2@polypyrrole magnetic nanocomposite and its application in the preconcentration of Cd(II) and Ni(II) ions. The parameters affecting the preconcentration procedure were optimised by a Box–Behnken design using response surface methodology. Three variables (extraction time, magnetic sorbent amount, and pH) were selected as the main factors affecting the sorption step, while four variables (type, volume and concentration of the eluent, and elution time) were selected as the main factors in the optimisation study of the elution step. Following the sorption and elution of analytes, the ions were quantified by FAAS. The limits of detection were 0.3 and 1.2 ng mL−1 for Cd(II) and Ni(II) ions, respectively. All the relative standard deviations were less than 8.8%. The obtained sorption capacities (in mg g−1) of this new sorbent were 120 for Cd(II) and 98 for Ni(II). Ultimately, this nanocomposite was successfully applied to the rapid extraction of trace quantities of heavy metal ions from seafood samples, and satisfactory results were obtained.

 Please wait while we load your content...
Please wait while we load your content...