A water soluble glucopyranosyl conjugate as a selective and reactive probe for cysteine in a buffer and its application to living cells†
Abstract
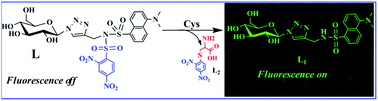
A water soluble and biocompatible glucopyranosyl conjugate (L) has been synthesized and characterized by various techniques. The L has been employed to recognize Cys selectively among the naturally occurring amino acids in HEPES buffer at physiological pH. A minimum detection limit of 2.5 × 10−7 M was shown by L for Cys in the buffer at pH = 7.4. The reactivity of L towards biological thiols as demonstrated by emission and absorption is supported by the observed increase in the fluorescence intensity; however, Cys shows a maximum increase owing to its better nucleophilicity. The reactivity of Cys on L is demonstrated by 1H NMR, ESI MS, emission and absorption spectroscopy, and the formation of the binary complex was supported by ESI MS. The control molecular study revealed the necessity of the glyco-moiety to bring water solubility and biological adaptability. The cellular studies support that the conjugate L is biologically adaptable and shows effective intracellular fluorescence emission upon reacting with intracellular thiols.

 Please wait while we load your content...
Please wait while we load your content...