Sequential separation of ultra-trace U, Th, Pb, and lanthanides using a simple automatic system
Abstract
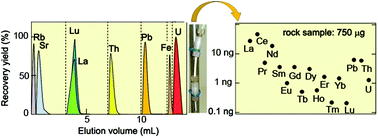
Uranium, thorium, lead, and the lanthanides were automatically and sequentially separated with a single anion-exchange column. This separation was achieved using eluents consisting of a simple and highly pure acid mixture of HCl, HNO3, acetic acid, and HF. The elements of interest were separated from the major constituents, which included alkaline metal elements, alkaline earth metal elements, and iron. This simple and automatic system is driven with pressurized nitrogen gas and controlled using a computer program. An optimized separation was accomplished under the following conditions: a 50 mm long and 2 mm diameter column, 11 μm diameter anion-exchange resin, and a 35 μL min−1 flow rate. Using this system, 50 ng of varied elements in a 100 μL feed solution were perfectly separated within 5 h with >400 decontamination factors and >95% yield. In order to evaluate the performance of this system, a reference powdered rock sample was separated using this system. Abundances of objective elements, including 0.23 ng of lutetium, were accurately determined without corrections of chemical recovery yield or subtraction of the process blank. This separation technique saves time and effort for chemical processing, and is useful for ultra-trace quantitative and isotopic analyses of elements in small environmental samples.

 Please wait while we load your content...
Please wait while we load your content...