Extremely supercharged proteins in mass spectrometry: profiling the pH of electrospray generated droplets, narrowing charge state distributions, and increasing ion fragmentation†
Abstract
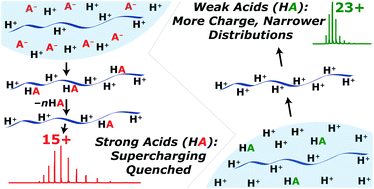
The effects of 12 acids, 4 solvents, and 8 low-volatility additives that increase analyte charging (i.e., superchargers) on the charge state distributions (CSDs) of protein ions in ESI-MS were investigated. We discovered that (i) relatively low concentrations [5% (v/v)] of 1,2-butylene carbonate (and 4-vinyl-1,3-dioxolan-2-one) can be added to ESI solutions to form higher charge states of cytochrome c and myoglobin ions than by using more traditional additives (e.g., propylene carbonate, sulfolane, or m-nitrobenzyl alcohol) under these conditions and (ii) the width of CSDs narrow as the effectiveness of superchargers increase, which concentrates protein ions into fewer detection channels. The use of strong acids (pKa values < 0) results in essentially no protein supercharging, higher adduction of acid molecules, and wider CSDs for many superchargers and proteins, whereas the use of weak acids (pKa > 0) results in significantly higher protein ion charging, less acid adduction, and narrower CSDs, indicating that protein ion supercharging in ESI can be significantly limited by the binding of conjugate base anions of acids that neutralize charge sites and broaden CSDs. The extent of protein charging as a function of acid identity (HA) does not strongly correlate with gas-phase proton transfer data (i.e., gas-phase basicity and proton affinity values for HA and A−), solution-phase protein secondary structures (as determined by circular dichroism spectroscopy), and/or acid molecule volatility data. For protein-denaturing solutions, these data were used to infer that the “effective” pH of ESI generated droplets near the moment of ion formation can be ∼0, which is ca. 1 to 3 pH units lower than the pH of the solutions prior to ESI. Electron capture dissociation (ECD) of [ubiquitin, 17H]17+ resulted in the identification of 223 cleavages, 74 of 75 inter-residue sites, and 92% ECD fragmentation efficiency, which correspond to highest of these values that have been obtained by ECD of a single isolated charge state of ubiquitin.

 Please wait while we load your content...
Please wait while we load your content...