A colorimetric nitrite detection system with excellent selectivity and high sensitivity based on Ag@Au nanoparticles†
Abstract
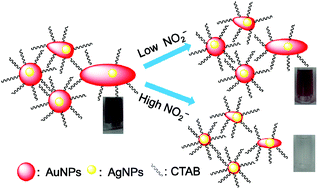
Excessive uptake of NO2− is detrimental to human health, but the currently available methods used to sensitively detect this ion in the environment are cumbersome and expensive. In this study, we developed an improved NO2− detection system based on a redox etching strategy of CTAB-stabilized Ag–Au core–shell nanoparticles (Ag@AuNPs). The detection mechanism was verified by UV-Vis spectroscopy, TEM and XPS. The detection system produces a color change from purple to colorless in response to an increase of NO2− concentration. The selectivity of detection of NO2−, both with the unaided eye and by measurement of UV-Vis spectra, is excellent in relation to other ions, including Cu2+, Co2+, Ni2+, Cr3+, Al3+, Pb2+, Cd2+, Ca2+, Ba2+, Zn2+, Mn2+, Mg2+, Fe3+, Hg2+, Ag+, K+, F−, PO43−, C2O42−, SO32−, CO32−, SO42−, NO3− and CH3–COO− (Ac−). The limit of detection (LOD) for NO2− is 1.0 μM by eye and 0.1 μM by UV-Vis spectroscopy. The LOD by eye is lower than the lowest previously reported value (4.0 μM). There is a good linear relationship between A/A0 and the concentration of NO2− from 1.0 to 20.0 μM NO2−, which permits a quantitative assay. The applicability of our detection system was also verified by analysis of NO2− in tap water and lake water. The results demonstrate that our Ag@AuNP-based detection system can be used for the rapid colorimetric detection of NO2− in complex environmental samples, with excellent selectivity and high sensitivity.

 Please wait while we load your content...
Please wait while we load your content...