Mechanistic studies on the reactivity of sensitizing allylic hydroperoxides: investigation of the covalent modification of amino acids by carbon-radical intermediates†
Abstract
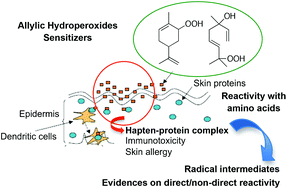
Many fragrance terpenes autoxidize yielding allylic hydroperoxides that are strong skin allergens. These chemicals are supposed to bind through radical reactions to proteins in the skin to start the immunotoxic process characterizing skin sensitization, and further allergic contact dermatitis. In order to get more insight into this reactivity, we investigated the chemical behavior of the allylic hydroperoxides responsible for the allergenicity of autoxidized limonene and linalool. We studied by liquid chromatography-mass spectrometry the reactivity in Fe(II)/Fe(III) systems, able to degrade peroxides in vivo, toward amino acids prone to radical reactions, together with glutathione. We validated that carbon radicals issued from these compounds alter directly the lateral chain of some of the amino acids tested forming adducts via radical processes. However, in parallel, due to the high oxidative strength of the media, we show that redox processes can also be induced and engender chemical modifications on the amino acids themselves. Amino acids chemically modified by the oxidant stress can then react with radicals issued from the hydroperoxides through what we call a “non-direct” reactivity process. Understanding protein binding processes to allergenic allylic hydroperoxides needs thus to consider both covalent binding of carbon radicals to the amino acids and additional oxido-reduction processes contributing to their sensitizing potential.

 Please wait while we load your content...
Please wait while we load your content...