The unusual electronic structure of ambipolar dicyanovinyl-substituted diketopyrrolopyrrole derivatives†
Abstract
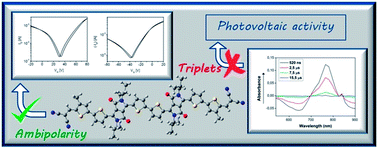
We have synthesized two novel dicyanovinylene-substituted DPP–oligothiophene semiconductors, DPP-4T-2DCV and 2DPP-6T-2DCV. In these materials, the combination of an extended oligothiophene conjugated skeleton with the strong electron-withdrawing DPP–dicyanovinylene groups results in semiconductors exhibiting ambipolar TFT response with reasonably balanced electron and hole mobilities of up to 0.16 cm2 V−1 s−1 and 0.02 cm2 V−1 s−1, respectively. Furthermore, no thermal annealing of the semiconductors is necessary to afford high mobility, making them ideal candidates for low cost fabrication of devices on inexpensive plastic foils. Analysis of the molecular and electronic structures by means of electronic and vibrational spectroscopy techniques, electrochemistry and DFT calculations highlights a unique electronic scenario in these semiconductors, where the external cyano groups are isolated from the π-conjugated core. The appearance of these unusual π-systems explains the similar electron mobilities recorded for both DPP-4T-2DCV and 2DPP-6T-2DCV, despite their different skeletal dimensions. Furthermore, it also supports the appearance of moderately balanced hole and electron mobilities in semiconductors with such large accumulation of acceptor units. Transient spectroscopy measurements indicate the appearance of triplet excited state species, which may be related to the semiconductors' low performances in OPVs, due to the intrusion of triplets in the carrier formation process.

 Please wait while we load your content...
Please wait while we load your content...