Weak intermolecular interactions promote blue luminescence of protonated 2,2′-dipyridylamine salts†
Abstract
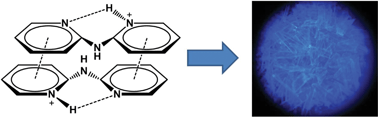
In this work we demonstrate that protonation and π-stacking can be exploited to convert non-luminescent 2,2′-dipyridylamine into blue-emitting derivatives. We have synthesized a series of luminescent 2,2′-dipyridylamine (dpa) salts, i.e., (dpaH)X·nSolv (dpa = 2,2′-dipyridylamine, X = HF2, n = 0.5, Solv = H2O 1; X = Cl, n = 2, Solv = H2O 2; X = Br, n = 2, Solv = H2O 3; X = I n = 1, Solv = H2O 4a; X = I n = 1, Solv = CHCl34b), (dpaH)2[SiF6]·H2O 5 and (dpaH)X (X = I36; SbF67; BF48) and characterized their emission properties, both in the solid-state and in solution. To rationalize our observations and relate the luminescence properties to the structure in the solid state and in solution, we have performed ab initio computations and showed that π-stacking interactions facilitate emission by sterically hindering radiation-less deactivation via a conical intersection. Our results thus not only provide a synthetic approach to cheap blue-emitting materials, but also revealed the essential role of weak intra- and intermolecular interactions, in particular hydrogen bonding and π-stacking, in the emission process. Our insights therefore provide new concepts for designing dpa-based systems with improved luminescence properties.

 Please wait while we load your content...
Please wait while we load your content...