Synthesis, properties, and applications of poly(ethylene glycol)-decorated tetraphenylethenes†
Abstract
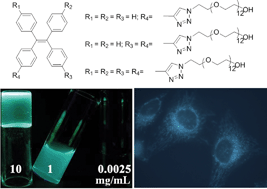
Non-charged, water soluble poly(ethylene glycol) (PEG)-decorated teraphenylethenes (TPEs) with different polymer chain numbers are synthesized in high yields by azide–alkyne cycloaddition. Their aggregation and thermosensitive behaviours are investigated by means of fluorescence spectroscopy, transmission electron microscopy (TEM), zeta potential and dynamic mechanical analyses. All the luminogens are non-emissive in solutions, but emit intensely when aggregated in aqueous solutions, or forming micelles, demonstrating a phenomenon of aggregation-induced emission. The TPE derivative (1) carrying one PEG chain forms hydrogels in the THF–water mixture depending on the concentration, water fraction and temperature. All the luminogens are thermosensitive, with their cloud point being tunable by varying the solvent composition and their hydrophilicity. Luminogen 1 is biocompatible and can function as a fluorescent visualizer for intracellular imaging.

 Please wait while we load your content...
Please wait while we load your content...