Roles of an oxygen Frenkel pair in the photoluminescence of Bi3+-doped Y2O3: computational predictions and experimental verifications
Abstract
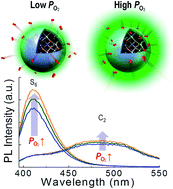
Bi3+ as a dopant in wide-band-gap yttria (Y2O3) has been used as a green light emission center or a sensitizer of co-doped rare earth elements. Because the photoluminescence (PL) properties of Y2O3:Bi3+ vary remarkably according to heat treatment, the roles of point defects have been an open question. By using first-principles calculations and thermodynamic modeling, we have thoroughly investigated the formation of point defects in Y2O3:Bi3+ at varying oxygen partial pressures and temperatures, as well as their roles in PL. The photoabsorption energies of the Bi3+ dopant were predicted to be 3.1 eV and 3.4 eV for doping at the S6 and the C2 sites, respectively, values that are in good agreement with the experimental values. It was predicted that an oxygen interstitial (Oi) and an oxygen vacancy (VO) are the dominant defects of Y2O3:Bi3+ at ambient pressure and an annealing temperature of 1300 K (3.19 × 1016 cm−3 for 1% Bi doping), and the concentrations of these defects in doped Y2O3 are approximately two orders of magnitude higher than those in undoped Y2O3. The defect 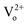 in Y2O3:Bi3+ was predicted to reduce the intensity of PL from Bi3+ at both S6 and C2 sites. We verify our computational predictions from our experiments that the stronger PL of both 410 and 500 nm wavelengths was measured for the samples annealed at higher oxygen partial pressure.
in Y2O3:Bi3+ was predicted to reduce the intensity of PL from Bi3+ at both S6 and C2 sites. We verify our computational predictions from our experiments that the stronger PL of both 410 and 500 nm wavelengths was measured for the samples annealed at higher oxygen partial pressure.

 Please wait while we load your content...
Please wait while we load your content...