Cross-linking of protein scaffolds for therapeutic applications: PCL nanofibers delivering riboflavin for protein cross-linking
Abstract
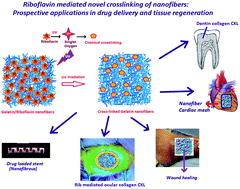
Nanofibers play a significant role in tissue engineering and drug delivery because of the ease with which drugs or pharmaceuticals may be incorporated into the nano-formulation. Natural protein nanofibers are cross-linked (CXLed) employing a new protocol to improve their stability for perspective usage as tissue engineering or drug delivery scaffolds. The protocol utilizes a non-toxic, natural material vitamin based CXL protocol that works well for stabilizing protein nanofibers. We have tested the generation of reactive oxygen species (ROS) from UV treated riboflavin–gelatin microfibers, film or solution that helps in gelatin (Gel) CXLing and results in improved mechanical properties. Further natural proteins Gel and fibrinogen (Fib) solutions were also CXLed using vitamin B2 (riboflavin (Rib)) released from Rib-loaded polycaprolactone (PCL) nanofibers followed by UV treatment. The sustained release of Rib from PCL nanofibers is studied with in vitro drug release experiments and in vitro hydrogel formation upon treatment with the natural protein solutions. Rib-loaded nanofibers were characterized with SEM and AFM for morphology, mechanical strength calculation and FT-IR for ensuring drug incorporation. The Rib encapsulation in the nanofiber reservoirs enables the sustained release, and the ROS generating nanofibers could find application as a patch for CXLing any protein fiber or fibrous tissue, such as ocular, skin or cardiac tissue engineering.

 Please wait while we load your content...
Please wait while we load your content...