Cyclometalated Ir(iii) complexes with styryl-BODIPY ligands showing near IR absorption/emission: preparation, study of photophysical properties and application as photodynamic/luminescence imaging materials†
Abstract
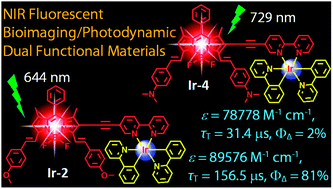
Heteroleptic C^N cyclometalated iridium(III) complexes incorporating a monostyryl/distyryl BODIPY ligand via acetylide bonds of 2,2′-bipyridine (bpy) with both absorption (ca. ε = 8.96 × 104 M−1 cm−1, 9.89 × 104 M−1 cm−1, and 7.89 × 104 M−1 cm−1 at 664 nm, 644 nm, and 729 nm for Ir-2, Ir-3 and Ir-4, respectively) and fluorescence emission bands (ca. 624–794 nm for Ir-1, Ir-2, Ir-3 and Ir-4) in the near infra-red region (NIR) and exceptionally long-lived triplet excited states (τ = 156.5 μs for Ir-2) have been reported. Ir(ppy)3 (Ir-0; ppy = 2-phenylpyridine) was used as reference, which gives the typical weak absorption in visible range (ε = 1.51 × 104 M−1 cm−1 M−1 cm−1 at 385 nm). The nanosecond time-resolved transient absorption and DFT calculations proposed that styryl BODIPY-localized long lived 3IL states were populated for Ir-1, Ir-2, Ir-3 and Ir-4 (τT = 106.6 μs, 156.5 μs, 92.5 μs and 31.4 μs, respectively) upon photoexcitation. The complexes were used as triplet photosensitizers for singlet oxygen (1O2) mediated photooxidation of 1,5-dihydronaphthalene to produce juglone. The 1O2 quantum yields (ΦΔ) of Ir-1 (0.53) and Ir-2 (0.81) are ca. 9-fold of Ir-3 (0.06) and 40-fold of Ir-4 (0.02), respectively. Ir-2 has high molar absorption coefficient at 664 nm, moderate fluorescence in the NIR region, and high singlet oxygen quantum yield (ΦΔ = 0.81), exhibits predominate photocytotoxicity over dark cytotoxicity in LLC cells (lung cancer cells) upon irradiation, making it potentially suitable for use in in vivo photodynamic therapy (PDT). Our results are useful for preparation of transition metal complexes that show strong absorption of visible light in the NIR region with long-lived triplet excited states and for the application of these complexes in photocatalysis and theranostics such as simultaneous photodynamic therapy (PDT) and luminescent bioimaging.
- This article is part of the themed collection: JMC B Top Picks web collection: Seeing the unseen: Advances in bioimaging and biosensors

 Please wait while we load your content...
Please wait while we load your content...