Carbazole linked phenylquinoline-based fullerene derivatives as acceptors for bulk heterojunction polymer solar cells: effect of interfacial contacts on device performance†
Abstract
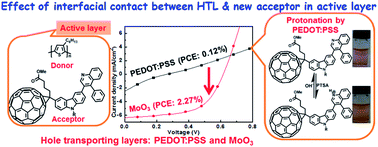
To understand the effect of interfacial contact between the hole transporting layer (HTL) and fullerene derivatives in the active layer of bulk heterojunction polymer solar cells (BHJ PSCs), carbazole (Cz) linked phenylquinoline (PhQ)-based fullerene derivatives, PhQHCz-C61BM and PhQEOCz-C61BM, have been successfully synthesized. They are used as acceptors with a poly(3-hexylthiophene) (P3HT) donor in the active layer, and PEDOT:PSS and MoO3 were used as the HTL. Both the derivatives are highly soluble in common organic solvents and possess high thermal stability. BHJ PSCs are fabricated with configurations of ITO/PEDOT:PSS/P3HT:PhQHCz-C61BM/LiF/Al, ITO/PEDOT:PSS/P3HT:PhQEOCz-C61BM/LiF/Al, and ITO/MoO3/P3HT:PhQHCz-C61BM/LiF/Al, ITO/MoO3/P3HT:PhQEOCz-C61BM/LiF/Al, and the device characteristics were measured under AM1.5G (100 mW cm−2). Both derivatives exhibited much lower power conversion efficiencies (PCE) of ∼0.1% when PEDOT:PSS was employed as the HTL. In contrast, the PCE increases to ∼2.2% upon replacing PEDOT:PSS with MoO3 as the HTL. This is due to the fact that protonation of the pyridyl nitrogen of the acceptor in the active layer by the –SO3H group of PEDOT:PSS in the HTL, establishes a charge injection barrier at the interfacial contact and leads to restricted charge collection at the electrodes. This was indirectly confirmed by protonation of pyridyl nitrogen in PhQHCz-C61BM by the –SO3H group in p-toluenesulphonic acid.

 Please wait while we load your content...
Please wait while we load your content...