Na2FePO4F cathode utilized in hybrid-ion batteries: a mechanistic exploration of ion migration and diffusion capability†
Abstract
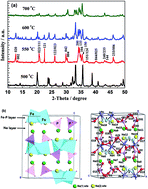
Layered Na2FePO4F is utilized as a cathode in hybrid-ion batteries in order to explore the ion migration and diffusion capability. It is the first time that the ion migration mechanism and capability in a hybrid-ion battery is investigated by considering the activation energies of different migration ways. It is proposed that a rapid ion exchange of Na+ ions on the Na(2) site of the crystal structure with Li+ ions can take place to produce the NaLiFePO4F phase and is firstly confirmed by first principle calculations. Li+ ion conduction in NaLiFePO4F is prone to be two-dimensional (2D) in the interlayer plane with an essentially restricted migration along the [010] direction for interlayer transport due to the much higher energy value (4.53 eV for sodium ion and 1.63 eV for lithium ion). Additionally, the 2D ways which need lower activation energies along [100] and [001] directions and the small volume variation during redox cycling are responsible for the large diffusion capability with a maximum magnitude of 10−10 cm2 s−1.

 Please wait while we load your content...
Please wait while we load your content...