Aluminum–air battery based on an ionic liquid electrolyte†
Abstract
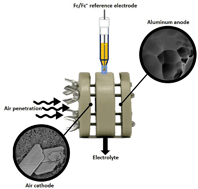
Metal–air batteries, and particularly aluminum–air (Al–air) batteries, draw a major research interest nowadays due to their high theoretical energy content of Al (gravimetric and volumetric). Nevertheless, the implementation of Al–air batteries as a sustainable energy storage device is hampered by severe hurdles. Al anode high corrosion rate in aqueous alkaline solution is of major concern in terms of Al usage and safety. In non-aqueous electrolytes adverse Al surface activation substantially limits any power output. This study presents a novel non-aqueous Al–air battery. This battery utilizes 1-ethyl-3-methylimidazolium oligo-fluoro-hydrogenate (EMIm(HF)2.3F) room temperature ionic liquid (RTIL). Al–air batteries can sustain current densities up to 1.5 mA cm−2, producing capacities above 140 mA h cm−2, thus utilizing above 70% of the theoretical Al capacity. This is equivalent to an outstanding energy densities of 2300 W h kg−1 and 6200 W h L−1. We detected Al2O3 at the air electrode as the battery discharge product of the oxygen reduction coupled with Al ions migrated to the air electrode.

 Please wait while we load your content...
Please wait while we load your content...