Enhanced activity, durability and anti-poisoning property of Pt/W18O49 for methanol oxidation with a sub-stoichiometric tungsten oxide W18O49 support†
Abstract
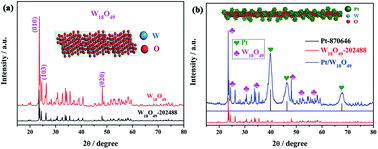
In this work, sub-stoichiometric tungsten oxide W18O49 was first studied as a support for a Pt catalyst. Metallic monoclinic W18O49 nanorods (NRs) with an isotropic morphology can not only improve electron, reactant, and product transport but can also enhance the utilisation of Pt for the methanol oxidation reaction (MOR). The specific activity for the forward peak (If) of Pt/W18O49 was determined to be 1.14 mA cmPt−2, which is approximately 1.4 and 1.8 times higher than that of Pt-black and Pt/C, respectively. On the other hand, the robust W18O49 NRs improve the stability of Pt/W18O49. After a 5000-cycle accelerated durability test, the electrochemical surface area (ECSA) loss rate of Pt/W18O49 was only 27.12%, less than that of Pt-black and Pt/C. Moreover, numerous oxygen vacancies and two valence states of W (W5+ and W6+) were found to co-exist in W18O49, which may promote hydrogen spillover and oxygen buffering. These effects together contributed to improve the anti-poisoning properties of Pt/W18O49 towards the intermediates in the MOR. The peak current ratio of the forward versus the backward (If/Ib) reaction is 1.12 for Pt/W18O49, 0.99 for Pt-black, and 0.79 for Pt/C. It was found that the Magnéli phase W18O49 may be a promising catalyst support for use in the MOR.

 Please wait while we load your content...
Please wait while we load your content...