Reducibility of Co at the La0.8Sr0.2CoO3/(La0.5Sr0.5)2CoO4 hetero-interface at elevated temperatures†
Abstract
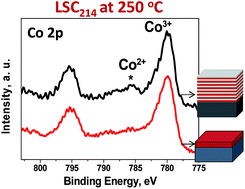
The fast kinetics of oxygen reduction reaction (ORR) at oxide hetero-structures made of La0.8Sr0.2CoO3 and (La0.5Sr0.5)2CoO4 (LSC113/214) attracted great interest to enable high performance cathodes for solid oxide fuel cells. The aim of this work is to uncover the underlying mechanism of fast ORR kinetics at the LSC113/214 system from a defect chemistry and electronic structure perspective. X-ray photoelectron spectroscopy with depth profiling was used to compare the reducibility of the Co cation and the valence band offset in the LSC113/214 multilayer (ML) and in single phase LSC113 and LSC214 films. At 250 °C, the Co 2p core-level photoelectron spectra showed the presence of Co2+ across the ML interfaces in both the LSC113 and LSC214 layers. While this is similar to the Co valence state of LSC113 single phase films, it is contrary to the single phase LSC214 films which had only the higher oxidation state of cobalt, Co3+. The greater reducibility of Co in LSC214 in the ML structure compared to that of Co in the LSC214 single phase film was attributed to electron donation and transfer of oxygen vacancies from LSC113 across the interfaces, and it is one mechanism to enhance the oxygen reduction activity at the LSC113/214 hetero-structure.

 Please wait while we load your content...
Please wait while we load your content...