Self-powered electrochemical deposition of Cu@Ni(OH)2 nanobelts for high performance pseudocapacitors†
Abstract
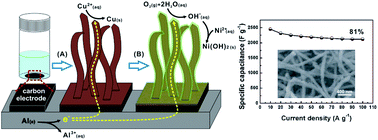
Two aluminum-driven spontaneous electrochemical reactions are developed for depositing Cu@Ni(OH)2 nanobelts on carbon/aluminum electrodes. The Cu@Ni(OH)2 NBs function as pseudocapacitor electrodes, which exhibit a high specific capacitance of 2426 F g−1 and a remarkable rate performance.

 Please wait while we load your content...
Please wait while we load your content...