Design of new electrode materials for Li-ion and Na-ion batteries from the bloedite mineral Na2Mg(SO4)2·4H2O†
Abstract
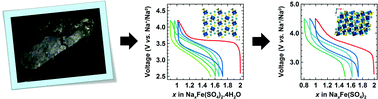
Mineralogy offers a large database to search for Li- or Na-based compounds having suitable structural features for acting as electrode materials, LiFePO4 being one example. Here we further explore this avenue and report on the electrochemical properties of the bloedite type compounds Na2M(SO4)2·4H2O (M = Mg, Fe, Co, Ni, Zn) and their dehydrated phases Na2M(SO4)2 (M = Fe, Co), whose structures have been solved via complementary synchrotron X-ray diffraction, neutron powder diffraction and transmission electron microscopy. Among these compounds, the hydrated and anhydrous iron-based phases show electrochemical activity with the reversible release/uptake of 1 Na+ or 1 Li+ at high voltages of ∼3.3 V vs. Na+/Na0 and ∼3.6 V vs. Li+/Li0, respectively. Although the reversible capacities remain lower than 100 mA h g−1, we hope this work will stress further the importance of mineralogy as a source of inspiration for designing eco-efficient electrode materials.

 Please wait while we load your content...
Please wait while we load your content...