3D core–shell architecture from infiltration and beneficial reactive sintering as highly efficient and thermally stable oxygen reduction electrode
Abstract
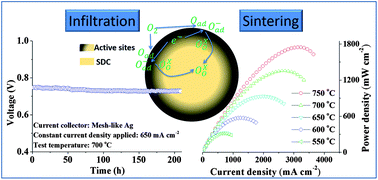
Solid oxide fuel cells (SOFCs) as alternatives for energy conversion have the capacity to overcome low energy conversion efficiency, highly detrimental emissions from traditional fuel utilization and the limited reserves of fossil fuels crisis. Herein, a 3D core–shell architecture has been fabricated from solution infiltration in combination with high-temperature reactive sintering and evaluated as the oxygen reduction electrode for SOFCs. The resultant electrode is composed of a stable porous Sm0.2Ce0.8O1.9 scaffold as the core for bulk oxygen ion diffusion, and a connective Sm,Ce-doped SrCoO3−δ perovskite film as the shell for efficient oxygen reduction reaction and partial current collection. The significant enhancement in conductivity, chemical and thermal compatibility with such core–shell structured electrodes can deliver promising and stable power outputs. An anode-supported solid oxide fuel cell with such a core–shell structured cathode exhibits a peak power density of 1746 mW cm−2 at 750 °C, which is comparable to the most promising cathodes ever developed. In addition, both a symmetrical cell and a fuel cell demonstrate favourable short-term stability during 200 h operation at 700 °C. The combined strategy involving infiltration and high-temperature reactive sintering (accompanied by ion diffusion) appears to be a promising approach to fabricate cathodes with high electrochemical performance and stability.

 Please wait while we load your content...
Please wait while we load your content...