Nanosized Mn–Ru binary oxides as effective bifunctional cathode electrocatalysts for rechargeable Li–O2 batteries
Abstract
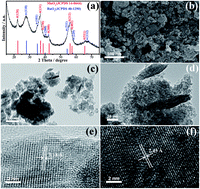
Mn–Ru binary oxides have been synthesized via a hydrothermal approach and their performance as bifunctional catalysts for the air electrode is evaluated in non-aqueous Li–O2 secondary batteries. Characterization of the catalysts by X-ray diffractometry and transmission electron microscopy confirms that the as-prepared oxides contain γ-MnO2 and hydrous RuO2 with the morphology of fusiform nanorods and nanoparticles, respectively. Linear scanning voltammetric measurements reveal that the binary oxides exhibit remarkable electrocatalytic activities towards both the oxygen reduction and oxygen evolution reactions. Li–O2 batteries with Mn–Ru oxides as cathode catalysts show a higher discharge voltage plateau and a higher specific capacity of 6500 mA h gcarbon−1 than those with Ketjen black (KB) alone, and the subsequent charge voltage is ca. 500 mV lower than that with KB. Moreover, much enhanced cyclability of Li–O2 batteries is achieved with a capacity retention ratio of 73.2% after 5 cycles. The cyclability is maintained for 50 cycles without sharp decay under a limited discharge depth of 1100 mA h gcarbon−1, suggesting that such a bifunctional electrocatalyst is a promising candidate for the air electrode in Li–air batteries.

 Please wait while we load your content...
Please wait while we load your content...