Self-limiting droplet fusion in ionic emulsions
Abstract
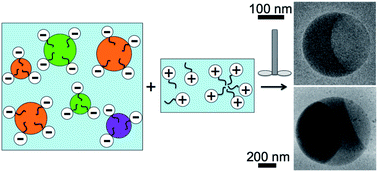
We make an oil-in-water emulsion, which is initially stabilized using a first ionic surfactant, and mix it with a solution of a second ionic surfactant having the opposite charge, thereby inducing massively parallel droplet fusion. A transient disruption of the screened-charge repulsive barrier between interacting droplets, caused by the second ionic surfactant, arises from significant yet temporary charge neutralization of the first ionic surfactant on the surfaces of the oil droplets while mixing occurs. Interestingly, if a moderate molar excess of one surfactant exists, then the resulting emulsion re-stabilizes after limited droplet fusion. By adjusting the droplet volume fraction, concentrations of first and second surfactants, and volumes of the emulsion and the solution of the second surfactant, we control the degree of droplet coalescence and achieve a self-limiting droplet fusion process. Using optical microscopy, we observe that flat, thin, crystalline films can form between the two oil compartments after fusion of two or more immiscible microscale droplets. However, no such crystalline films are seen on the highly curved oil–oil interfaces inside nanoscale droplets that are composed of two or more immiscible oils and have been fused in the same manner, as revealed by cryogenic transmission electron microscopy.

 Please wait while we load your content...
Please wait while we load your content...