Dicationic organic salts: gelators for ionic liquids†
Abstract
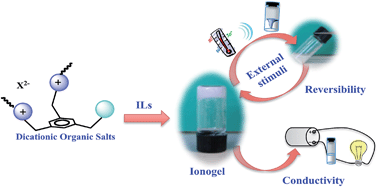
Diimidazolium and dipyrrolidinium organic salts were tested for their ability to gel both organic solvents and ionic liquids. Organic salts containing 1-(1-imidazolylmethyl)-3,5-di-(3′-octylimidazolylmethyl)-benzene and 1-(N-pyrrolidylmethyl)-3,5-di-(N,N-octylpyrrolidylmethyl)-benzene cations were used. In addition to the simple bromide anion, also dianions having a naphthalene core such as 1,5- and 2,6-naphthalenedisulfonate and 2,6-naphthalenedicarboxylate were taken into account. Gelation tests demonstrated that organic salts used were able to harden ionic liquids. The materials obtained were investigated for their thermal stability and also for electric conductivity properties using micro-DSC and dielectric spectroscopy. Furthermore, the opacity of some gel phases was monitored using UV-vis measurements. To obtain information about the gelation mechanism, gel phase formation was studied as a function of time by means of resonance light scattering investigation. Finally, the ability of materials to respond to external stimuli such as magnetic stirring or ultrasound irradiation was also analyzed. Data collected show that different relationships exist among the gelator and the ionic liquid structure, determining the properties of materials and their possible applications.

 Please wait while we load your content...
Please wait while we load your content...