The effect of antagonistic salt on a confined near-critical mixture
Abstract
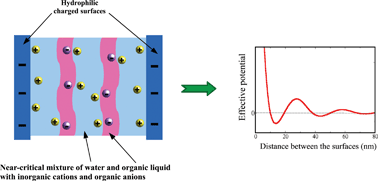
We consider a near-critical binary mixture with addition of antagonistic salt (hydrophilic cations and hydrophobic anions) confined between weakly charged and selective surfaces. A mesoscopic functional for this system is developed from a microscopic description by a systematic coarse-graining procedure. The functional reduces to the Landau–Brazovskii functional for amphiphilic systems for a sufficiently large ratio between the correlation length in the critical binary mixture and the screening length. Our theoretical result agrees with the experimental observation [Sadakane et al., J. Chem. Phys., 2013, 139, 234905] that the antagonistic salt and the surfactant both lead to a similar mesoscopic structure. For very low salt concentration ρion the Casimir potential is the same as in the presence of inorganic salt. For larger ρion the Casimir potential takes a minimum followed by a maximum for separations of order of tens of nanometers, and exhibits an oscillatory decay very close to the critical point. For separations of tens of nanometers the potential between surfaces with a linear size of hundreds of nanometers can be of order of kBT. We have verified that in the experimentally studied samples [Sadakane et al., J. Chem. Phys., 2013, 139, 234905, Leys et al., Soft Matter, 2013, 9, 9326] the decay length is too small compared to the period of oscillations of the Casimir potential, but the oscillatory force could be observed closer to the critical point.

 Please wait while we load your content...
Please wait while we load your content...