The position of hydrophobic residues tunes peptide self-assembly†
Abstract
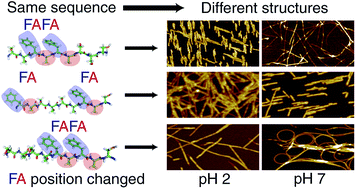
The final structure and properties of synthetic peptides mainly depend on their sequence composition and experimental conditions. This work demonstrates that a variation in the positions of hydrophobic residues within a peptide sequence can tune the self-assembly. Techniques employed are atomic force microscopy, transmission electron microscopy and an innovative method based on surface acoustic waves. In addition, a systematic investigation on pH dependence was carried out by utilizing constant experimental parameters.

 Please wait while we load your content...
Please wait while we load your content...