Effects of hydrophobic interaction strength on the self-assembled structures of model peptides
Abstract
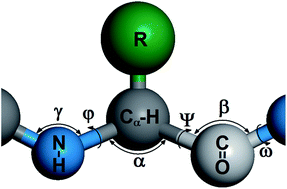
Stable and ordered self-assembled peptide nanostructures are formed as a result of cooperative effects of various relatively weak intermolecular interactions. We systematically studied the influence of hydrophobic interaction strength and temperature on the self-assembly of peptides with a coarse-grained model by Monte Carlo simulations. The simulation results show a rich phase behavior of peptide self-assembly, indicating that the formation and morphology of peptide assemblies may be tuned by varying the temperature and the strength of hydrophobic interactions. There exist optimal combinations of temperature and hydrophobic interaction strength where ordered fibrillar nanostructures are readily formed. Our simulation results not only facilitate the understanding of the self-assembly behavior of peptides at the molecular level, but also provide useful insights into the development of fabrication strategies for high-quality peptide fibrils.

 Please wait while we load your content...
Please wait while we load your content...