The impact of nonionic surfactant additives on the nonequilibrium association between oppositely charged polyelectrolytes and ionic surfactants†
Abstract
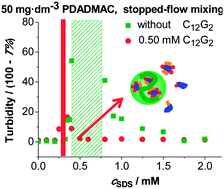
The effect of uncharged surfactant additives on the oppositely charged polyion/ionic surfactant complexation is usually described as a direct equilibrium association between the polyelectrolyte molecules and free mixed micelles analogous to the polyion/colloidal particle interactions. This approach predicts that the binding of the ionic surfactant to the polyelectrolyte molecules can be completely suppressed by increasing the nonionic-to-ionic surfactant ratio. In the present work, it is shown that the addition of nonionic surfactants to poly(diallyldimethylammonium chloride)/sodium dodecyl sulfate mixtures considerably enhances the binding of the anionic surfactant to the polycation in the dilute surfactant concentration regime. The dynamic light scattering, turbidity, electrophoretic mobility and fluorescence spectroscopic measurements are consistent with the synergic binding of the ionic and nonionic surfactants to the polyelectrolyte molecules. The enhanced surfactant binding could be utilized for the preparation of stable colloidal dispersions of novel polyion/mixed surfactant nanoparticles over a wide composition range provided that adequate mixing protocols are used. These results clearly indicate that the nonionic surfactant additives can be successfully used to tune the nonequilibrium association of oppositely charged macromolecules and amphiphiles.

 Please wait while we load your content...
Please wait while we load your content...