Bridging interactions of proteins with silica nanoparticles: The influence of pH, ionic strength and protein concentration†
Abstract
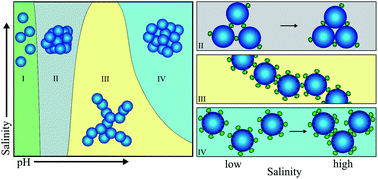
Charge-driven bridging of nanoparticles by macromolecules represents a promising route for engineering functional structures, but the strong electrostatic interactions involved when using conventional polyelectrolytes impart irreversible complexation and ill-defined structures. Recently it was found that the electrostatic interaction of silica nanoparticles with small globular proteins leads to aggregate structures that can be controlled by pH. Here we study the combined influence of pH and electrolyte concentration on the bridging aggregation of silica nanoparticles with lysozyme in dilute aqueous dispersions. We find that protein binding to the silica particles is determined by pH irrespective of the ionic strength. The hetero-aggregate structures formed by the silica particles with the protein were studied by small-angle X-ray scattering (SAXS) and the structure factor data were analyzed on the basis of a short-range square-well attractive pair potential (close to the sticky-hard-sphere limit). It is found that the electrolyte concentration has a strong influence on the stickiness near pH 5, where the weakly charged silica particles are bridged by the strongly charged protein. An even stronger influence of the electrolyte is found in the vicinity of the isoelectric point of the protein (pI = 10.7) and is attributed to shielding of the repulsion between the highly charged silica particles and hydrophobic interactions between the bridging protein molecules.

 Please wait while we load your content...
Please wait while we load your content...