Hexagonal closest-packed spheres liquid crystalline phases stabilised by strongly hydrated counterions†
Abstract
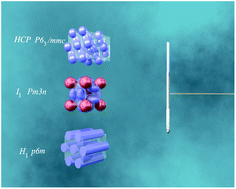
The sequence and structure of lyotropic liquid crystals formed in C12–C16 alkyltrimethylammonium surfactants with hydrolysable and multivalent phosphate (PO43−, HPO42− and H2PO4−), oxalate (HC2O4− and C2O42−), and carbonate (HCO3−/CO32−) counterions were determined using a concentration gradient method coupled with polarising optical microscopy and small angle X-ray scattering. In addition to the discrete cubic (I1, space group Pm3n) and hexagonal (H1, p6m) phases, almost all of these surfactants also formed the (previously) rare hexagonally closest-packed spheres (HCPS, P63/mmc) phase at compositions between the Pm3n cubic and L1 micellar phases. This structure has not been previously observed in cationic surfactants, but is readily achieved by using strongly hydrated counterions to stabilise spherical micelles at high concentrations.

 Please wait while we load your content...
Please wait while we load your content...