Polyelectrolyte adsorption, interparticle forces, and colloidal aggregation
Abstract
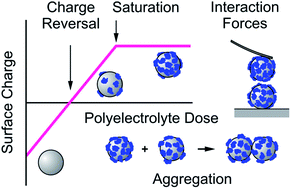
This review summarizes the current understanding of adsorption of polyelectrolytes to oppositely charged solid substrates, the resulting interaction forces between such substrates, and consequences for colloidal particle aggregation. The following conclusions can be reached based on experimental findings. Polyelectrolytes adsorb to oppositely charged solid substrates irreversibly up to saturation, whereby loose and thin monolayers are formed. The adsorbed polyelectrolytes normally carry a substantial amount of charge, which leads to a charge reversal. Frequently, the adsorbed films are laterally heterogeneous. With increasing salt levels, the adsorbed mass increases leading to thicker and more homogeneous films. Interaction forces between surfaces coated with saturated polyelectrolyte layers are governed at low salt levels by repulsive electric double layer interactions, and particle suspensions are stable under these conditions. At appropriately high salt levels, the forces become attractive, principally due to van der Waals interactions, but eventually also through other forces, and suspensions become unstable. This situation can be rationalized with the classical theory of Derjaguin, Landau, Verwey, and Overbeek (DLVO). Due to the irreversible nature of the adsorption process, stable unsaturated layers form in colloidal particle suspensions at lower polyelectrolyte doses. An unsaturated polyelectrolyte layer can neutralize the overall particle surface charge. Away from the charge reversal point, electric double layer forces are dominant and particle suspensions are stable. As the charge reversal point is approached, attractive van der Waals forces become important, and particle suspensions become unstable. This behaviour is again in line with the DLVO theory, which may even apply quantitatively, provided the polyelectrolyte films are sufficiently laterally homogeneous. For heterogeneous films, additional attractive patch–charge interactions may become important. Depletion interactions may also lead to attractive forces and suspension destabilization, but such interactions become important only at high polyelectrolyte concentrations.

 Please wait while we load your content...
Please wait while we load your content...