Pd(quinox)-catalyzed allylic relay Suzuki reactions of secondary homostyrenyl tosylates via alkene-assisted oxidative addition†
Abstract
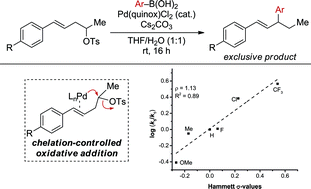
Pd-catalyzed allylic relay Suzuki cross-coupling reactions of secondary alkyl tosylates, featuring a sterically-hindered oxidative addition and precise control of β-hydride elimination, are reported. The identification of a linear free energy relationship between the relative rates of substrate consumption and the electronic nature of the substrate alkene suggests that the oxidative addition requires direct alkene involvement. A study of the effect of alkyl chain length on the reaction outcome supports a chelation-controlled oxidative addition.

 Please wait while we load your content...
Please wait while we load your content...