Nanoscale self-assembled multivalent (SAMul) heparin binders in highly competitive, biologically relevant, aqueous media†‡
Abstract
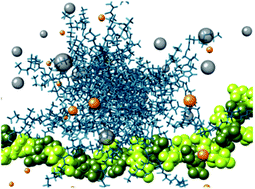
This paper investigates small molecules that self-assemble to display multivalent ligand arrays for heparin binding. In water, the self-assembled multivalent (SAMul) heparin binder is highly competitive with the current clinical heparin reversal agent, protamine. On addition of salt, the dimensions of the self-assembled nanostructure increase. This unique feature is due to the dynamic, responsive nature of assembly, predicted using multiscale modelling and proven experimentally, enhancing heparin binding of SAMul systems relative to fixed covalent multivalent nanostructures. Conversely, the presence of serum adversely affects the heparin binding of SAMul systems relative to covalent nanostructures due to partial destabilisation of the assemblies. Nonetheless, clotting assays in human plasma demonstrate that the SAMul system acts as a functional heparin reversal agent. Compound degradation, inducing nanostructure disassembly and loss of SAMul binding, takes place over 24 hours due to ester hydrolysis – but when bound to heparin, stability is enhanced. Heparin reversal in plasma, and the therapeutically useful degradation profile, make this SAMul approach of potential therapeutic value in replacing protamine, which has a number of adverse effects when used in the clinic.

 Please wait while we load your content...
Please wait while we load your content...