Supramolecular control of selectivity in transition-metal catalysis through substrate preorganization
Abstract
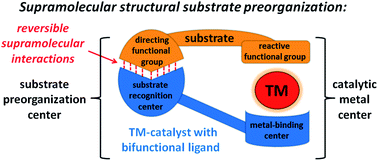
Supramolecular chemistry exploits multiple weak intermolecular interactions to assemble nano-sized molecular architectures, providing new possibilities for (transition metal) catalyst development. In this Perspective we focus on the application of such weak (directional) interactions between a substrate molecule and a (bifunctional) catalyst for structural preorganization prior to the catalytic reaction. As we discuss, such effects together with the confinement properties of the nano-space of the ‘active sites’ play a crucial role for the exceptional selectivities and activities of natural enzymes. We will elaborate on the application of such supramolecular strategy to the more traditional transition-metal catalysis, and we will compare it with the traditional substrate preorganization methods. Subsequently, literature examples of such bifunctional catalyst systems will be described in which the function of weak interactions was carefully designed a priori, as well as, the serendipitously found catalysts in which the presence of supramolecular effects was recognized post factum. The discussed examples demonstrate the power of the strategy for the control of selectivity in various types of metal catalyzed reactions, and the observation of the serendipitous findings can help to generate new leads for more efficient catalyst design.

 Please wait while we load your content...
Please wait while we load your content...