Transition metal-catalyzed direct nucleophilic addition of C–H bonds to carbon–heteroatom double bonds
Abstract
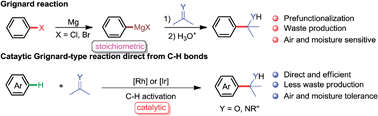
Transition metal-catalyzed direct C–H functionalization has drawn great attention in the past several decades owing to its advantages compared to conventional organic transformations, including higher atom-, step- and cost-economy and the avoidance of tedious prefunctionalization and waste emission. At the current stage, to make the C–H functionalization more applicable, chemists have devoted themselves to expanding the substrate and reaction scope. In the past decade, we exerted ourselves to develop new transformations based on direct C–H functionalization. In this minireview we report on our recent achievements on the addition of C–H bonds to carbonyls and imines. The addition of organometallic reagents, such as Grignard reagents, toward carbon–heteroatom double bonds is one of the most powerful reactions in organic synthesis to produce secondary and tertiary alcohols and amines. This chemistry is broadly used in both laboratory and industry. However, this powerful transformation suffers from some drawbacks: (1) the preparation of initial organohalides from easily available fossil feedstocks is tedious and sluggish; (2) substantial amounts of metal halide salts are emitted as waste; (3) last but not least, the manipulation of organometallic reagents is complicated due to their sensitivity to air and moisture. In contrast, direct insertion of polar double bonds to C–H bonds via transition-metal catalysis is ideal from the viewpoint of atom-, step- and cost-economy and the avoidance of the waste emission, as well as of the complex manipulation of sensitive reagents. Starting from this point, we made a commitment to this project years ago and have made credible achievements in this field. We first carried out Ir-catalyzed addition of pyridinyl C–H bonds to aldehydes promoted by silane, showing an unusual C-3 selectivity. Later on, we developed Rh-catalyzed addition of aryl C–H bonds with aldimines in the absence of any additives with directing strategy with highest atom- and step-economy. The mechanism was investigated in depth by the isolation of key intermediates and systematic thermodynamic and kinetic studies. Such a concept was expanded to the coupling of aryl/alkenyl C–H bonds with aldehydes and imines. Notably, a tandem process of relayed C–H activation/alkyne insertion/cyclization between benzoates/benzimide and alkynes was developed, indicating the potential of the direct coupling of esters and amides with C–H bonds. Ideally, this strategy opens a new window to approach the ideal reactions to produce amines and alcohols from hydrocarbons.

 Please wait while we load your content...
Please wait while we load your content...