Programmed synthesis of arylthiazoles through sequential C–H couplings†
Abstract
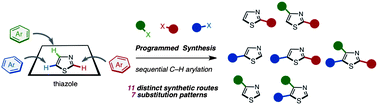
A programmed synthesis of privileged arylthiazoles via sequential C–H couplings catalyzed by palladium or nickel catalysts has been accomplished. This versatile protocol can supply all possible arylthiazole substitution patterns (2-aryl, 4-aryl, 5-aryl, 2,4-diaryl, 2,5-diaryl, 4,5-diaryl, and 2,4,5-triaryl) from an unfunctionalized thiazole platform by 11 distinct synthetic routes. We have generated over 150 arylthiazoles by using this methodology. We have applied this method to the rapid synthesis of fatostatin (SREBP inhibitor), and the gram-scale synthesis of triarylthiazoles has been demonstrated.

 Please wait while we load your content...
Please wait while we load your content...