Development and investigation of a site selective palladium-catalyzed 1,4-difunctionalization of isoprene using pyridine–oxazoline ligands†
Abstract
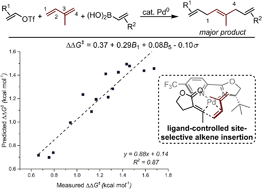
Palladium-catalyzed 1,4-difunctionalizations of isoprene that produce skipped polyenes are reported. Complex isomeric product mixtures are possible as a result of the difficult-to-control migratory insertion of isoprene into a Pd–alkenyl bond, but good site selectivity has been achieved using easily accessible pyrox ligands. Mechanistic studies suggest that the control of insertion is the result of the unique electronic asymmetry and steric properties of the ligand.

 Please wait while we load your content...
Please wait while we load your content...