Formal asymmetric hydrobromination of styrenes via copper-catalyzed 1,3-halogen migration†
Abstract
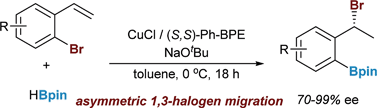
An enantioselective Cu(I)-catalyzed 1,3-halogen migration reaction accomplishes a formal hydrobromination by transferring a bromine activating group from a sp2 carbon to a benzylic carbon in good er and with concomitant borylation of the Ar–Br bond. Computational modelling aids in understanding the reaction outcome and suggests future directions to improve the formal asymmetric hydrobromination. The benzyl bromide can be displaced with a variety of nucleophiles to produce a wide array of functionalized products.

 Please wait while we load your content...
Please wait while we load your content...