Base catalytic activity of alkaline earth MOFs: a (micro)spectroscopic study of active site formation by the controlled transformation of structural anions†
Abstract
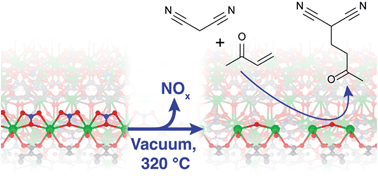
A new method has been developed for generating highly dispersed base sites on metal–organic framework (MOF) lattices. The base catalytic activity of two alkaline earth MOFs, M2(BTC)(NO3)(DMF) (M = Ba or Sr, H3BTC = 1,3,5-benzenetricarboxylic acid, DMF = N,N-dimethylformamide) was studied as a function of their activation procedures. The catalytic activity in Knoevenagel condensation and Michael addition reactions was found to increase strongly with activation temperature. Physicochemical characterization using FTIR, 13C CP MAS NMR, PXRD, XPS, TGA-MS, SEM, EPR, N2 physisorption and nitrate content analysis shows that during activation, up to 85% of the nitrate anions are selectively removed from the structure and replaced with other charge compensating anions such as O2−. The defect sites generated via this activation act as new strong basic sites within the catalyst structure. A fluorescence microscopic visualization of the activity convincingly proves that it is exclusively associated with the hexagonal crystals, and that reaction proceeds inside the crystal's interior. Theoretical analysis of the Ba-material shows that the basicity of the proposed Ba2+–O2−–Ba2+ motifs is close to that of the edge sites in BaO.

 Please wait while we load your content...
Please wait while we load your content...