Oxygen chemisorption/desorption in a reversible single-crystal-to-single-crystal transformation†
Abstract
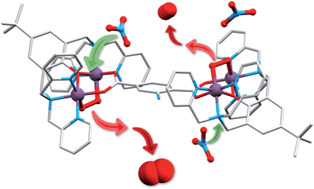
Crystalline salts of a series of cationic multimetallic cobalt complexes reversibly, selectively and stoichiometrically chemisorb dioxygen in a process involving the two electron oxidation of dimetallic sites with concurrent reduction of two equivalents of sorbed O2 to form μ-η1,η2-peroxide ligands. The coordinating ability of counteranions, ClO4−, PF6−, BF4−, CF3SO3− and NO3− determine the O2 affinity of the deoxygenated forms, and the nitrate and triflate salts sorb dioxygen at a significantly slower rate compared to the PF6− and BF4− salts (hours versus sub-seconds at ambient temperature and pressure). Single crystal X-ray structural determination for a nitrate salt of the 2-aminoterephthalato-linked deoxy system, [{(bpbp)Co2II(NO3)}2(NH2bdc)](NO3)2·2H2O (bpbp− = 2,6-bis(N,N-bis(2-pyridylmethyl)aminomethyl)-4-tert-butylphenolato, NH2bdc2− = 2-amino-1,4-benzenedicarboxylato) shows that nitrate ions are coordinated as bridging ligands. These crystals undergo reversible single-crystal-to-single-crystal (SC-to-SC) transformations on the stoichiometric uptake of O2. During this process O2 replaces the two nitrate ligands. Thus the Co ions are six coordinated in both the oxy and deoxy states. This SC-to-SC process involves the concerted fast migration of neutral dioxygen through the crystal lattice and the translational movement by 4–6 Å of at least two of nitrate anions. Rapid hydration/dehydration processes involving several molecules of co-crystallized water per unit cell accompany the reaction. Besides large atom movements involving O2, NO3− and H2O, these impressive examples of consecutive SC-to-SC-to-SC transformations involve the cleavage of four bonds, and the creation of four new bonds, in one single molecule. The solid state structural rearrangements observed provide an explanation for the slower rates of dioxygen uptake for the complexes isolated as nitrate salts, and by inference, the triflate salts, compared to the salts of more weakly coordinating counteranions, ClO4−, PF6− and BF4−.


 Please wait while we load your content...
Please wait while we load your content...